EHR will be a digital system that holds a person’s full health and social care information in one place. It will replace paper files and local IT systems by allowing staff to record, update and access all health information in one place.
Access to digital health records has been a long-sought objective in Irish healthcare for many years.
To date(March 25), the Health Service has rolled out a number of electronic health record (EHR) systems in different sites in recent years. These include the National Rehabilitation Hospital, the National Forensic Mental Health Service, St. James Hospital, Mater Misericordiae University Hospital, roll-out across the bigger Maternity Hospitals extending EHR coverage to 70% of births nationally by the end of 2025. The National Children’s Hospital EHR deployment will be the most comprehensive EHR deployment in the state when the hospital is commissioned.
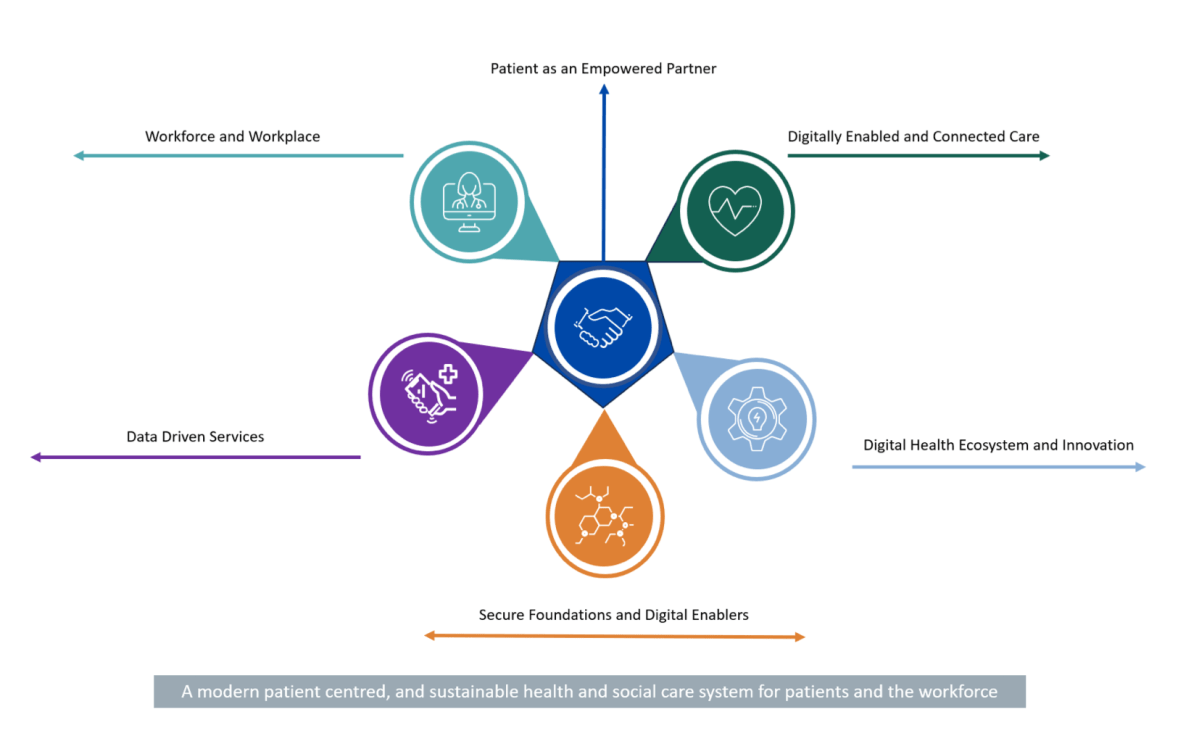
Ireland needs one digital health record for every citizen that can be access by health professionals across the service.
To achieve this, the HSE are following a three-step approach: delivering the HSE Health App, developing a National Shared Care Record (NSCR), and regional deployments of enterprise level Electronic Health Record systems that span acute and community healthcare.
HSE has completed the procurement for the National Shared Care Record (NSCR) programme and has now been mobilised, with the contract for building the NSCR technology platform awarded to EY, Better and Kainos.
The NSCR brings together healthcare information from various sources such as hospitals, GP practices, and Community care into a single place, making them available at the point of care and self-care in read only format. By having access to key healthcare information in one place means healthcare professionals will be able to make more informed, safer decisions and to focus more time on direct patient care while patients will be better informed and empowered to manage their own healthcare.
A phased roll-out of the national shared care record is due to commence in Q4 2025 in the South-East region with University Hospital Waterford. The system will then extend to other regions from 2026 with additional information being added over time.
https://www.oireachtas.ie/en/debates/question/2025-03-04/696/